Peach (Prunus persica L.)
Peach (Prunus persica L.) belongs to the Rosaceae family, originated in China. Different varieties of peaches are highly consumed worldwide. It is commercially grown in many countries at a fair range of different climate conditions and types of soils.
Peach fruits are a drupe composed of skin (epidermis), flesh (mesocarp), and stone (endocarp) enclosing the seed. Ripening peach can be clingstone (flesh adhering to the stone) or freestone (flesh separating from the stone); melting (flesh quickly softening) or non-melting (flesh remaining firm). The flesh can be white, yellow or red according to mesocarp colour.
Peaches present many secondary metabolites, such as phenolic compounds, carotenoids and tocopherols that present important biological actions and are associated with disease prevention. Two main groups of phenolic compounds present in peach have different structural characteristics – NonFlavonoids, which include phenolic acids; and Flavonoids, which include flavonols, flavan-3-ols and anthocyanins.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]
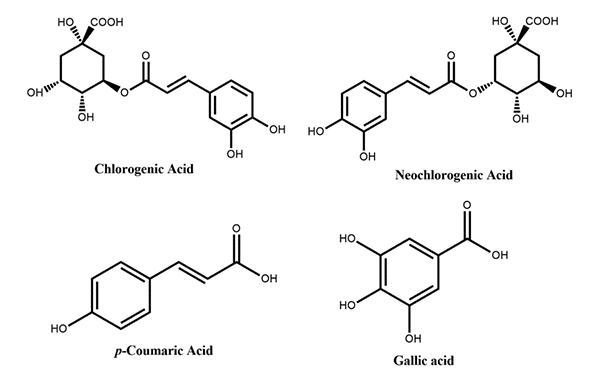 Figure 1: Phenolic acids reported in peach
Figure 1: Phenolic acids reported in peach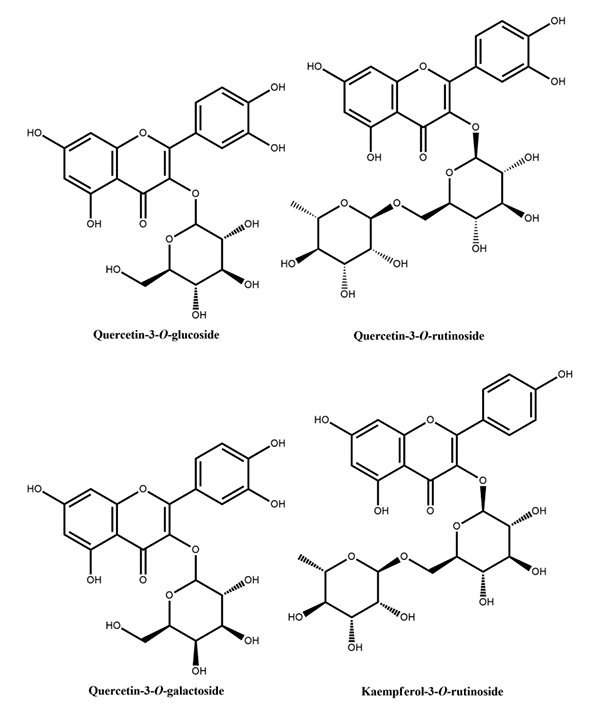 Figure 2: Flavanols reported in peach
Figure 2: Flavanols reported in peach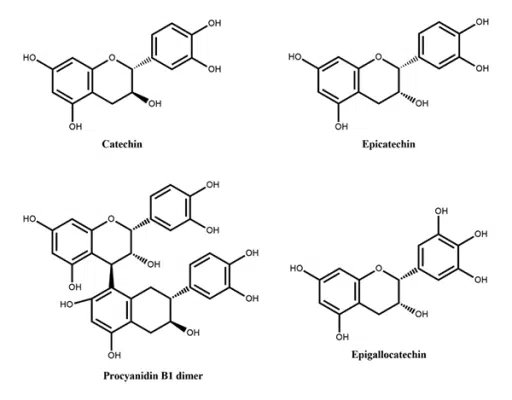 Figure 3: Flavan-3-ols reported in peach
Figure 3: Flavan-3-ols reported in peach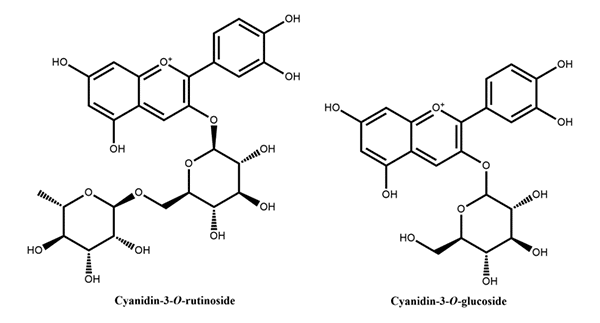 Figure 4: Anthocyanins reported in peach
Figure 4: Anthocyanins reported in peachWe provide practical, easy & consistent on food processing application.
Fresh natural flavor extract powder for Bakery & Beverage.
Vegan, Non-GMO, No Fillers, No Flavors, No Additives, No Artificial Colors, Soy and Gluten Free, Lab Tested for Authentic & Active Compound
Anti-tumor
| In vivo study was conducted to investigate tumor growth inhibition and anti-metastatic effects of peach phenolics | ||
|---|---|---|
| Subject | Female athymic nude mice implanted with MDA-MB-435 human breast cancer cells. 10 days after tumor implantation, animals were divided into equal groups (5 per group) | |
| Duration | 24 days | |
| Group and Dosage | Xenograft group Group 1 – received 100µL 10% saline solution (xenografted control) Group 2 – received Peach phenolics (0.2 to 1.6 mg chlorogenic acid equivalents (CAE)/day dissolved in saline solution) by oral gavageNon-Xenografted group Group 1 – received 0.8 CAE/day Group 2 – received 1.6 mg CAE/day |
|
| Parameters analyzed |
|
|
| Outcomes |
Visible benefits
|
|
| Functions | Peach phenolics inhibit tumor growth and protect against angiogenesis and metastasis. | |
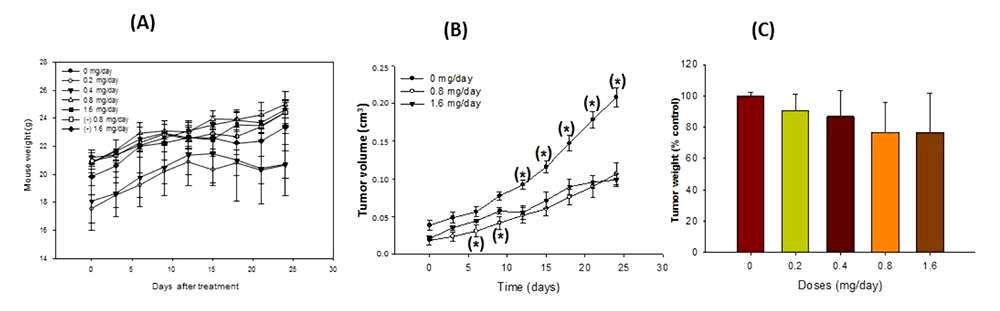
Figure 5: Anti-tumorigenic activity of peach phenolics in xenografted nude mice. Effects on body weight (A), tumor volume (B) and tumor weight (C)

Figure 6: mRNA levels of metalloproteinases (MMPs) MMP-1, MMP-2, MMP13, and MMP-3 in peach phenolics-treated mice (0.8 – 1.6 mg CAE/day)
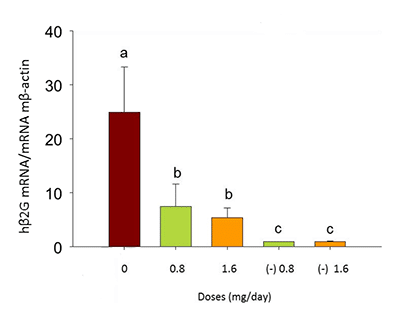
Figure 7: Expression of human-specific hβ2G in the lungs of xenografted and non-xenografted control mice treated with peach phenolics (0.8 – 1.6 CAE/day)
| Materials |
Lyophilized samples of:
|
| Subject | Liver, kidney and brain cortex tissue slices from rats were pre-incubated with peach samples, after that incubated with a ferrous sulphate and hydrogen peroxide (FeSO4 /H2O2) system in order to induce cytotoxicity, producing hydroxyl radicals. |
Antioxidant
| Parameter Analyzed | Result | |
|---|---|---|
| In Vitro Study | Total reactive antioxidant potential (TRAP assay) indicates the quantity of antioxidants present in the plant extract |
The peel suspension showed the highest antioxidant activity, followed by the FPP suspension. PPP had no significant effect |
| Total antioxidant reactivity (TAR) indicates antioxidant effectiveness |
Peel and FPP had the highest TAR indexes, compared to PPP and syrup. | |
| Ex Vivo Study | Level of lactate dehydrogenase (LDH) indicates cytotoxicity |
|
| Antioxidant enzymes – Superoxide Dismutase (SOD) and Catalase (CAT) monitor oxidative stress |
|
|
| Quantification of total reduced sulfhydryl (SH) groups oxidative status of thiol groups |
|
|
| Formation of carbonyl groups oxidative damage to proteins |
|
|
| Formation of thiobarbituric acid reactive species (TBARS) oxidative damage on lipids |
|
|
| Function Fresh peach pulp and oeel demonstrated high antioxidant effects preventing against cytotoxicity, oxidative stress and oxidative damage towards lipids and proteins.Peach peels presented antioxidant activity mainly in the lipid fraction while Fresh peach pulp had a major antioxidant effect to soluble protein fractions suggest that different secondary compounds present in distinct parts of the fruit (i.e., pulp and peel) are responsible for these effects. | ||
Anti Glycation
| In vitro study | ||
|---|---|---|
| Parameter | Isolated albumin subjected to a glycation protocol through incubation with glucose and fructose during 21 days | |
| Result | Albumin glycation was significantly inhibited by peel and FPP by 40% at different doses. PPP only by 10%, while the syrup alone, probably due to its high sucrose content (more than 20%), enhanced glycation by 30%. | |
| Function | Fresh peach pulp and peel reduce effect of protein glycation | |
Anti-inflammatory
| Ex vitro study | ||
|---|---|---|
| Parameter | Quantification of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) | |
| Result |
|
|
| Function | Fresh peach pulp and peel showed anti-inflammatory effect | |
[/vc_column_text][vc_column_text]
Figure 8: Antioxidant and anti-glycation profile
(A) Total reactive antioxidant potential (TRAP assay)
(B) Kinetics of chemiluminescence intensity
(C) TAR index
(D) Percentage of in vitro albumin glycation[/vc_column_text][vc_column_text]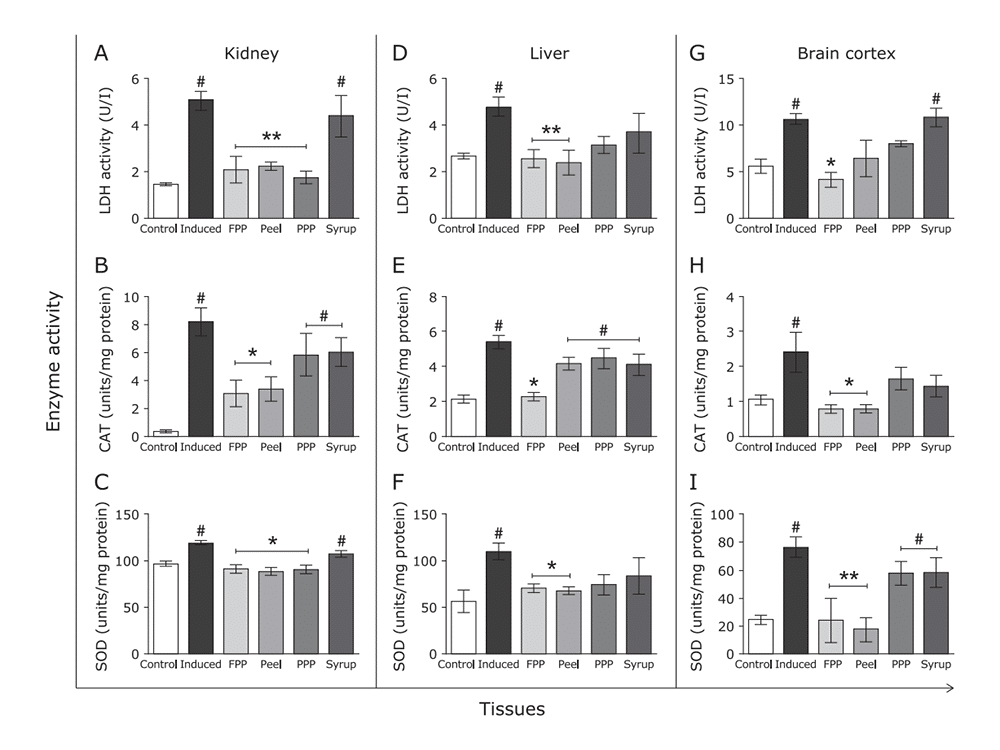
Figure 9: Effects of FPP, peel, PPP and syrup on cytotoxicity and antioxidant enzyme activities in tissue slices subjected to oxidative stress
(A) LDH activity in the incubation medium was analyzed as a parameter for cytotoxicity (cell rupture).
(B) CAT and (C) SOD activities were assessed in homogenized tissue slices of kidney.
(D), (E), (F) represent respectively the same protocols to liver homogenate, and (G), (H), and (I) to brain cortex.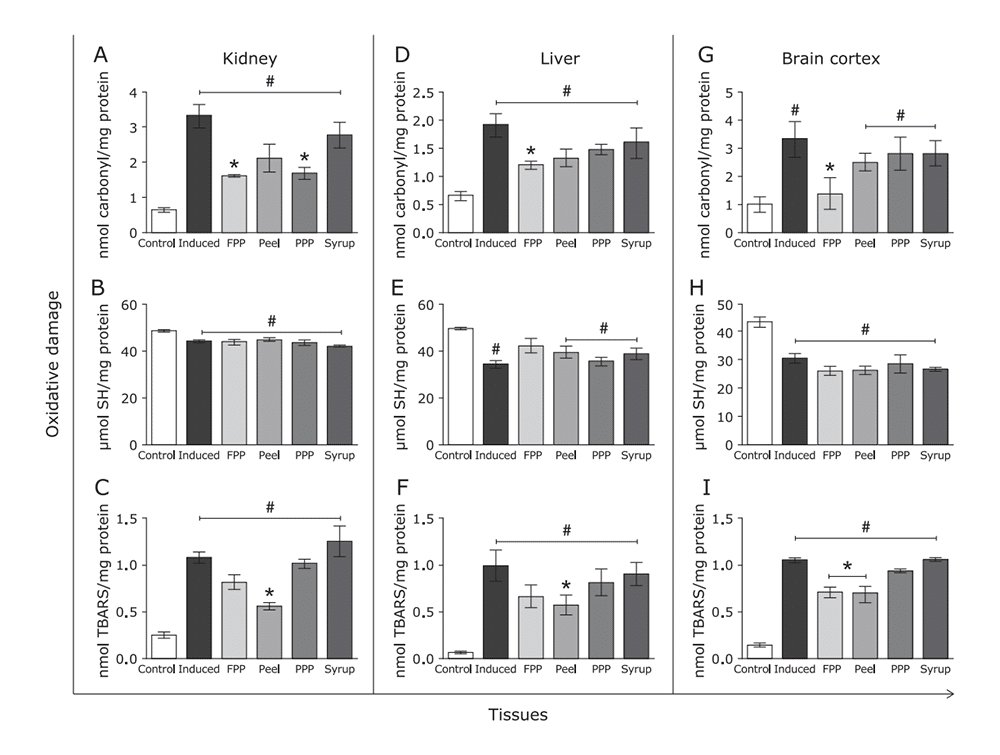
Figure 10: Effects of FPP, peel, PPP and syrup on biomolecule oxidative damage. Tissues were homogenized and analyzed for (A), (B), (C) protein carbonylation, reduced sulphydryl content and TBARS content in kidney. The same assays were conducted to liver (D), (E), (F) and brain cortex (G), (H), (I).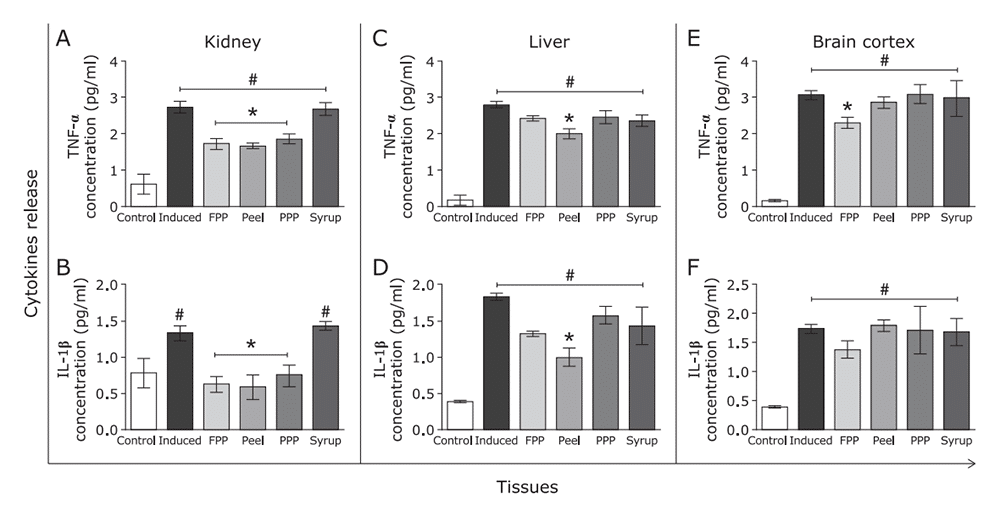
Figure 11: Effects of FPP, peel, PPP and syrup on interleukin release. (A) TNF-α of kidney, (C) liver and (E) brain cortex was quantified. IL1-β levels in (B) kidney, (D) liver and (F) brain cortex was evaluated.
Reference
- Catarina Bento, Ana C. Gonçalves, Branca Silva, Luís R. Silva. (2020) Peach (Prunus Persica): Phytochemicals and Health Benefits. Food Reviews International, 38 (8), 1703-1734
DOI:10.1080/87559129.2020.1837861 - Mokrani, A, Krisa, S, Cluzet, S, Costa, G.D, Temsamani, H., Renouf, E., Mérillon, J.M., Madani, K., Mesnil, M., Monvoisin, A. et al. (2016) Phenolic contents and bioactive potential of peach fruit extracts. Food Chemistry. 202, 212-220.
DOI: 10.1016/j.foodchem.2015.12.026 - Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. (2015) Phenolic Composition and Antioxidant Properties of Different Peach [Prunus Persica (L.) batsch] Cultivars in China. . Int. J. Mol. Sci., 16(3), 5762–5778.
DOI: 10.3390/ijms16035762. - Dabbou S, Maatallah S, Castagna A, Guizani M, Sghaeir W, Hajlaui H, Ranieri A. (2017) Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Foods for Human Nutrition, 72, 103-110
DOI: 10.1007/s11130-016-0585-y - G. Noratto, W. Porter, D. Byrne, and L. Cisneros-Zevallos (2014) Polyphenolics from peach (Prunus persica var. Rich Lady) inhibit tumor growth and metastasis of MDA-MB-435 breast cancer cells in vivo. The Journal of Nutritional Biochemistry, 25 (7): 796–800
DOI: 10.1016/j.jnutbio.2014.03.001 - Gasparotto J, Somensi N, Bortolin RC, et al. (2014) Effects of different products of peach (Prunus persica L. Batsch) from a variety developed in southern Brazil on oxidative stress and inflammatory parameters in vitro and ex vivo. Journal of Clinical Biochemistry and Nutrition, 55(2):110-119
DOI: 10.3164/jcbn.13-97

Peach (Prunus persica) Standardized Extract Powder
- Pure 20:1 Peach Prunus persica Extract
- Good for the eyes
- Healthy for heart
- Healthy for skin
- Help in weight loss
- Improve digestion
- May detoxify your system
- May help reduce stress
- Rich in antioxidant
- Ultrasonic hot water/solvent extraction
























